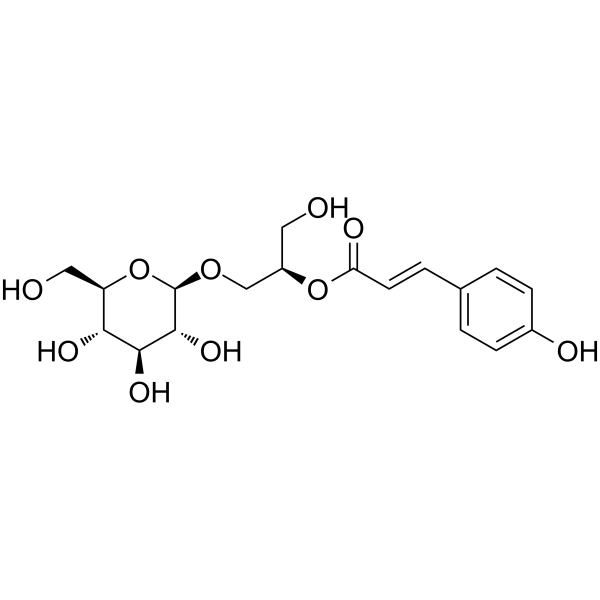
Regaloside H
CAS No. 126239-77-8
Regaloside H( —— )
Catalog No. M29431 CAS No. 126239-77-8
Regaloside H, a glyceryl phenylpropionate glycoside, is a gluconeogenesis inhibitor.
Purity : >98% (HPLC)
 COA
COA
 Datasheet
Datasheet
 HNMR
HNMR
 HPLC
HPLC
 MSDS
MSDS
 Handing Instructions
Handing Instructions
| Size | Price / USD | Stock | Quantity |
| 5MG | 291 | Get Quote |


|
| 10MG | 432 | Get Quote |


|
| 25MG | 707 | Get Quote |


|
| 50MG | 963 | Get Quote |


|
| 100MG | Get Quote | Get Quote |


|
| 200MG | Get Quote | Get Quote |


|
| 500MG | Get Quote | Get Quote |


|
| 1G | Get Quote | Get Quote |


|
Biological Information
-
Product NameRegaloside H
-
NoteResearch use only, not for human use.
-
Brief DescriptionRegaloside H, a glyceryl phenylpropionate glycoside, is a gluconeogenesis inhibitor.
-
DescriptionRegaloside H, a glyceryl phenylpropionate glycoside, is a gluconeogenesis inhibitor.(In Vitro):Administration of Regaloside H inhibited glucose production in H4IIE rat hepatoma cells by 36.8% .
-
In VitroRegaloside H (10 μM) suppresses the glucose production by 36.8% in H4IIE rat hepatoma cells.
-
In Vivo——
-
Synonyms——
-
PathwayOthers
-
TargetOther Targets
-
Recptor——
-
Research Area——
-
Indication——
Chemical Information
-
CAS Number126239-77-8
-
Formula Weight400.38
-
Molecular FormulaC18H24O10
-
Purity>98% (HPLC)
-
SolubilityIn Vitro:?DMSO : 100 mg/mL (249.76 mM)
-
SMILESOC[C@H](CO[C@@H]1O[C@H](CO)[C@@H](O)[C@H](O)[C@H]1O)OC(=O)\C=C\c1ccc(O)cc1
-
Chemical Name——
Shipping & Storage Information
-
Storage(-20℃)
-
ShippingWith Ice Pack
-
Stability≥ 2 years
Reference
molnova catalog



related products
-
MDL-28170
Calpain Inhibitor III is a potent, cell-permeable inhibitor of calpain I and II (Ki = 8 nM)., The Calpain Inhibitor III, also referenced under CAS 88191-84-8, controls the biological activity of Calpain.
-
Salvianolic acid E
Salvianolic acid E is a natural product from Salvia miltiorrhiza Bge.
-
Fmoc-Gly-Gly-Phe-OtB...
Fmoc-Gly-Gly-Phe-OtBu is a cleavable ADC linker used in the synthesis of antibody-drug conjugates (ADCs).



 Cart
Cart
 sales@molnova.com
sales@molnova.com


